
According to World Health Organization (WHO), cervical cancer is one of the most serious threats to women’s lives. By various estimates, there are more than a million women suffering from this malignant disease worldwide. In 2020 alone, cervical cancer took the lives of more than 342,000 women.
In 2020, WHO approved the Global Strategy for cervical cancer elimination. The main goals of the program, called “90-70-90”, will completely save humanity from the problem of cervical cancer within the next 100 years. This is a 90% vaccination rate of the human papillomavirus (HPV) for girls under the age of 15 (before the sexual activity begins); accessibility for at least 70% of women of reproductive age to highly effective methods of screening (diagnosis) of cervical cancer and, finally, providing treatment for at least 90% of women diagnosed with cervical disease. [1]
The main aim that we set ourselves when writing this article is to once again remind the available and recommended methods for screening cervical pathology, and share our best practices in this direction.
The Problem
The generally recognized types of cervical screening, i.e., methods for the primary diagnosis of cervical pathology are visual inspection with acetic acid (VIA); cytology (PAP smear test), and HPV testing. Moreover, in all cases where a cervical pathology is detected during a screening study, a confirmation of the clinical diagnosis by colposcopic examination and/or taking a targeted cervical biopsy is required. That is why, in achieving the targets set by WHO, the solution of two main tasks at the national and global levels is of key importance. Firstly, this is a solution to the problem of accessibility of wide female population groups to effective methods for diagnosing cervical pathology. Secondly, the professional training level of specialists conducting relevant examinations.
From our point of view, the problems that women in the target group have with the opportunity to gain access to cervical screening are primarily due to the fact that the vast majority of women with cervical cancer, according to the WHO statistics, lived (and live now!) in low- and middle-income countries. So about 90% of the total number of cases of cervical cancer are registered in the countries of Central and South America, East Africa, South, and Southeast Asia, and the Western Pacific. [2]
The second most difficult problem to implement is the maximum objectification of the chosen diagnostic method.
In this regard, in the area of our interest lies the objectification of a visual method for diagnosing cervix pathology, such as a colposcopic examination. We agree with the thesis that during the period of maximum objectification of the processed data, colposcopy is a dying art but it remains an integral part of measures to prevent cervical cancer from ever developing. So, in the United States, colposcopy continues to be widely used in various medical settings, including academic and non-academic institutions, primary health care settings in urban and rural areas, and by the way, continues to be funded by both private and public resources [3]. The study itself is usually examined by experienced specialists (65% physicians, 36% advanced practice clinicians (APC), i.e., nurse practitioners and paramedics). [4]
Nevertheless, colposcopy is a rather subjective diagnostic method that requires training and constant practice of using the method to obtain the appropriate competencies. Thus, according to the scientific literature, the frequency of false-negative results (missed squamous intraepithelial / high-grade malignancy invasive cancer) during colposcopy ranges from 13% to 69%. [5, 6]
That is why attempts are being made to improve the diagnostic properties of colposcopy by developing new additional technologies that use the biochemical and morphological changes present in dysplastic (atypical) cells of the cervix.
There are three main areas of modern developments: fluorescence and reflectance spectroscopy, dynamic spectral imaging, and optical coherence tomography. In spite of that, it is worth emphasizing that only one of them, namely dynamic spectral imaging, has received FDA approval and is sold in the United States.
Our Solution
Having analyzed the abovementioned information, we have focused our efforts specifically on the development of an effective and available screening methodology that will help women who do not have the opportunity to undergo face-to-face screening in a medical institution, for example, women living in remote and/or areas that are difficult to access for modern medical technologies.
Our proposed method of primary/intermediate screening of cervical pathology can be represented by the following scheme:
patient -> device that allows a woman to take a picture of the cervix without outside help -> Internet access -> website/application -> double assessment of the resulting image: a medical expert and our artificial intelligence algorithm -> personal recommendation for the patient.
At this stage, we can already offer a prototype of our device, which has passed the experimental research stage and showed impressive results. Intravaginal system “Lily” with a working camera and backlight.

The machine learning model developed by our team is called “Dr. Gynecom”. It is trained with the Keras framework and TensorFlow backend. According to the scientific requirements, a model with two outputs was chosen – for segmentation and classification of the input image. The U-Net architecture was taken as a basis, where the decoder is a common part for both outputs.
Segmentation detects the location of pathologies, and classification indicates the accuracy of the presence of a particular pathology shown in the processed image. Thus, the application draws the doctor’s attention to problematic areas of the cervix with the result of two separate branches of the model algorithm, while the patient is able to receive a personally verified conclusion and a recommendation. The model was trained on the basis of 1500 colposcopic images marked by a high-category practice doctor. Various augmentation algorithms were used to increase the dataset. As a result, the accuracy of the trained data for segmentation exceeds 90.5% and 96.5% – for classification.
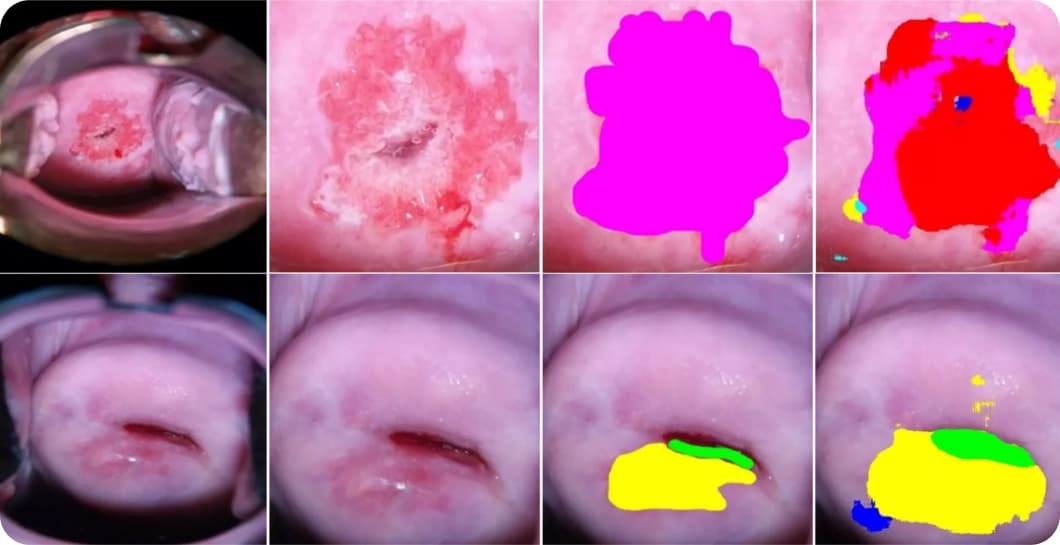
We invite you to see the results of our work at Dr. GyneCom website .
Literature
- Global strategy to accelerate the elimination of cervical cancer as a public health problem; 17 November 2020. https://www.who.int/publications/i/item/9789240014107
- World Cancer Report. Edited by Stewart BW, Wild CP. Lyon, France: International Agency for Research on Cancer, World Health Organization; 2014.
- Mayeaux EJ, Novetsky AP, Chelmow D, Choma K, Garcia F, Liu AH, Papasozomenos T, Einstein MH. Systematic Review of International Colposcopy Quality Improvement Guidelines. J Low Genit Tract Dis. 2017 Oct;21(4):249-257.
- ASCCP Colposcopy Standards: How Do We Perform Colposcopy? Implications for Establishing Standards. Alan G Waxman 1, Christine Conageski, Michelle I Silver, Candice Tedeschi, Elizabeth A Stier, Barbara Apgar, Warner K Huh, Nicolas Wentzensen, L Stewart Massad, Michelle J Khan, Edward J Mayeaux Jr, Mark H Einstein, Mark H Schiffman, Richard S Guido. J Low Genit Tract Dis. 2017 Oct;21(4):235-241.
- Davies KR, Cantor SB, Cox DD, Follen M. An alternative approach for estimating the accuracy of colposcopy in detecting cervical precancer. PLoS One. 2015;10(5):e0126573.
- Wentzensen N, Walker JL, Gold MA, Smith KM, Zuna RE, Mathews C, Dunn ST, Zhang R, Moxley K, Bishop E, Tenney M, Nugent E, Graubard BI, Wacholder S, Schiffman M. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015 Jan 01;33(1):83-9.
- Павлюченко М. І. Акушерство і гінекологія. Фонові, передракові та злоякісні захворювання шийки матки : навчальний посібник для самостійної роботи студентів IV, VI курсів медичних факультетів спеціальностей «Медицина», «Педіатрія» / М. І. Павлюченко. – Запоріжжя : ЗДМУ, 2021. – 125 с.
